simaerep
Simulate adverse event reporting in clinical trials with the goal of detecting under-reporting sites.
Monitoring of Adverse Event (AE) reporting in clinical trials is important for patient safety. We use bootstrap-based simulation to assign an AE under-reporting probability to each site in a clinical trial. The method is inspired by the ‘infer’ R package and Allen Downey’s blog article: “There is only one test!”.

Adverse Events
An adverse event (AE) is any untoward medical occurrence in a patient or participating in a clincial trial. These events are not necessarily drug related. It could anything from a headache to a sporting accident to a life-threatening pneunomia. It is important though for the safety of the patients that these events are being reported in a timely manner from the clinical trial site to the sponsor of the trial so that the safety profile of the drug can be updated if necessary and appropriate actions can be taken.
{simaerep}
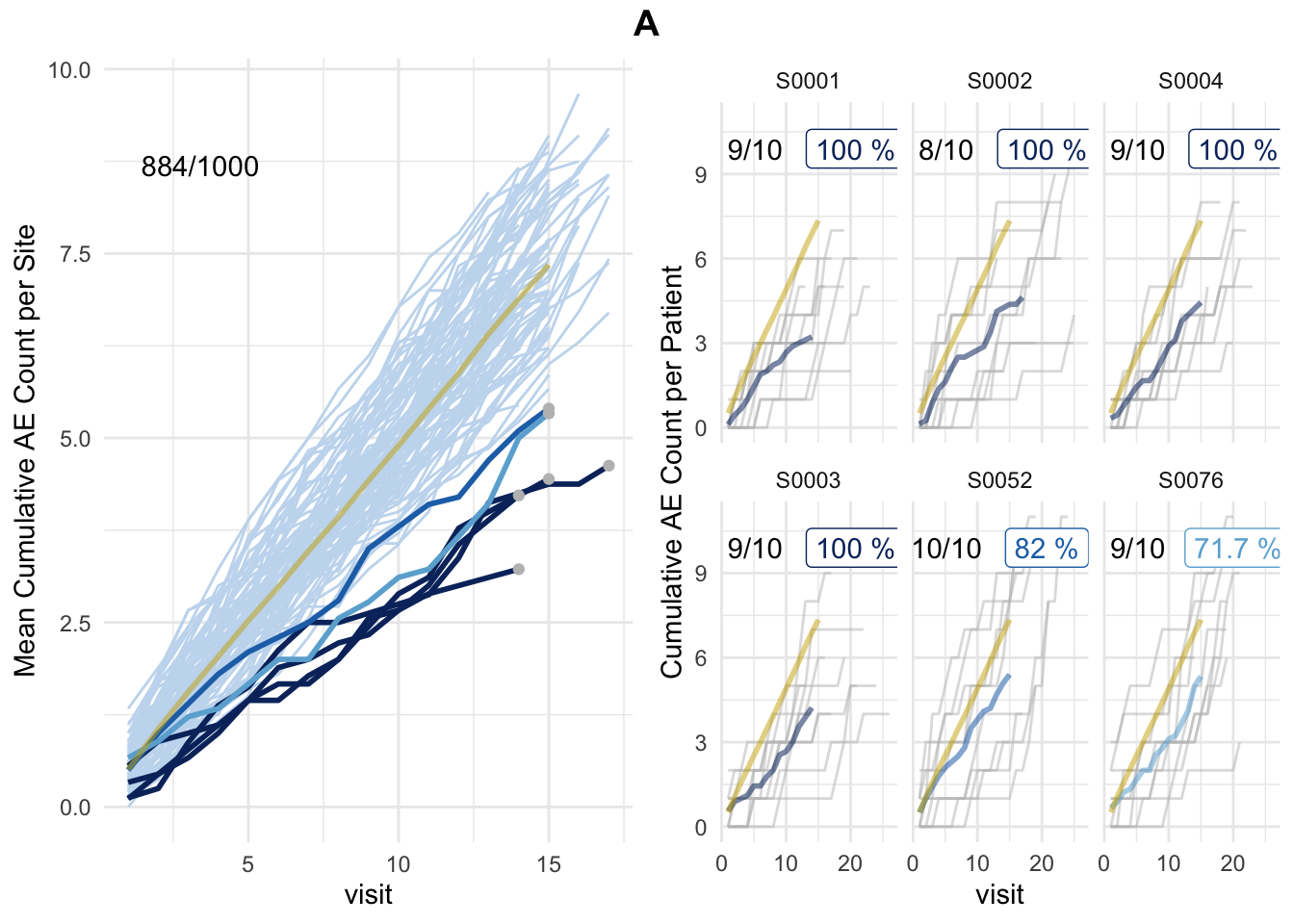
simaerep can be used to flag sites that are statistically reporting fewer AEs than other sites by using bootstrap resampling. For a site that needs to be tested it draws with replacement a new patient sample from the entire study patient population. This is repeated several times to be able to determine the probability to get an equal or lower number of AEs than initially reported.
There is a bit more to it of course simaerep needs to account for the fact that patients have been recruited at different points in time and it needs to account for the alpha-error that occurs when you perform many statistical tests. The exact methodology is explained here
AE Reporting Quality Assurance
simaerep is currently a cornerstone in our AE reporting quality assurance strategy. As we describe in our latest publication:
Koneswarakantha, B., Barmaz, Y., Ménard, T. et al. Follow-up on the Use of Advanced Analytics for Clinical Quality Assurance: Bootstrap Resampling to Enhance Detection of Adverse Event Under-Reporting. Drug
Saf (2020).
https://doi.org/10.1007/s40264-020-01011-5
Collaboration
We are frequently refining our quality analytics methods and are happy to collaborate in developing industry standards. Please reach out if you have any comments or questions.
Application
suppressPackageStartupMessages(library("simaerep"))
set.seed(1)
df_visit <- sim_test_data_study(
n_pat = 1000, # number of patients in study
n_sites = 100, # number of sites in study
frac_site_with_ur = 0.05, # fraction of sites under-reporting
ur_rate = 0.4, # rate of under-reporting
ae_per_visit_mean = 0.5 # mean AE per patient visit
)
df_visit$study_id <- "A"
df_site <- site_aggr(df_visit)
df_sim_sites <- sim_sites(df_site, df_visit, r = 1000)
df_eval <- eval_sites(df_sim_sites, r_sim_sites = 1000)
plot_study(df_visit, df_site, df_eval, study = "A")